Published
Arch Sex Behav. 1985 Feb;14(1):29-40.
Abstract
The effects of oral estrogen treatment on sexual physiology and behavior were examined in seven presurgical male-to-female transsexuals engaged in cross-living. Subjects were studied prior to hormone treatment, during long-term hormone treatment, and during an experimental double-blind period in which the effects of their usual hormone regimen were compared to those of placebo during successive 4-week periods. Subjects maintained daily logs of their spontaneous erections, sexual activity (masturbation), and feelings throughout the study. Nocturnal penile tumescence was measured, using home monitors, in order to estimate estrogen-induced changes in erectile capacity. Erectile response to sexually arousing stimuli (erotic films and self-generated fantasy) was also assessed in the laboratory. Blood samples were taken at intervals for testosterone and sex-hormone-binding globulin measurements and free testosterone levels were calculated. Estrogen treatment inhibited sexual activity, spontaneous erections, and nocturnal penile tumescence. No significant effects on psychophysiological response to film and fantasy or frequency of sexual feelings were found, but the psychophysiological data were very variable. Testosterone levels were suppressed by estrogen, but not to the extent that free testosterone levels were. It appears that declining free testosterone level is associated with inhibition of spontaneous erections (during both sleep and waking) and of sexual activity, though the latter relationship is less clear. No evidence of an effect on film or fantasy-induced erections was obtained.
INTRODUCTION
It is now well established that testosterone has multiple stimulatory effects on sexual behavior both in male mammals (Davidson 1977; Davidson et al., 1978) and men (Davidson et al., 1979,1982; Skakkebaek et al., 1980). Yet much remains to be learned about the behavioral mechanism of this action of androgen, particularly in men, since controlled human research on this subject is still meager (Bancroft, 1980; Davidson et al. 1982).
We recently showed that testosterone stimulates nocturnal penile tumescence in hypogonadal men in addition to increasing sexual activity and spontaneous erections, but erections elicited in the laboratory during psychophysiological testing were not affected by hypogonadism or testosterone treatment (Kwan et al., 1983). That some types of erection might be androgen-dependent, and others not, seems counterintuitive, and further investigation is indicated. A different approach to these problems is to study the effects of androgen suppression in healthy men. The availability of presurgical male-to-female transsexuals undergoing hormone therapy provides an opportunity to study the effects of estrogen simultaneously on sexual behavior and testosterone levels in physically normal genetic males and to test the hypothesis that estrogen's suppressive effects would parallel testosterone inhibition. Unfortunately, uniform experimental protocols could not be maintained across these individually variable subjects, who are usually highly invested in their treatment regimens. Nevertheless, we were able to gather informative experimental and clinical data on effects of estrogen treatment in healthy men and to compare the results with findings in a companion study of effects of testosterone treatment on components of sexuality in hypogonadal men (Kwan et al., 1983).
METHODS
Seven genetically male transsexual subjects (see Table I) were recruited for long-term assessment and a double-blind crossover study of estrogen vs. placebo. They were referred from the Gender Dysphoria Program (directed by D. Laub, M.D.), a multidisciplinary project that has provided diagnosis and treatment for approximately 750 transsexuals. All were living as women during a mandatory 2-3-year period of cross-dressing and daily estrogen ingestion before qualifying for gender change surgery. Because of the reluctance of these patients to change or relinquish their estrogen treatments, even for a short period, their recruitment required a long-term establishment of trust with the investigative team. Moreover, the patients' previous oral estrogen treatment regimen was used in the blind experiment. Two subjects took the informed consent option of having the code broken and returning to estrogen treatment if a subject suffered undue disquiet while on placebo, 2 weeks (subject 1) and 3 weeks (subject 5) after onset of placebo treatment. Since in these two cases the estrogen was administered first, these (and the other) experiments were blind during estrogen treatment. The subjects were seen by the senior author at regular intervals over extended periods of time before, during, and after the blind experimental period. Total and free plasma testosterone levels and self-report and physiological data on sexual behavior (see below) were obtained from the subjects periodically, and results are reported here.
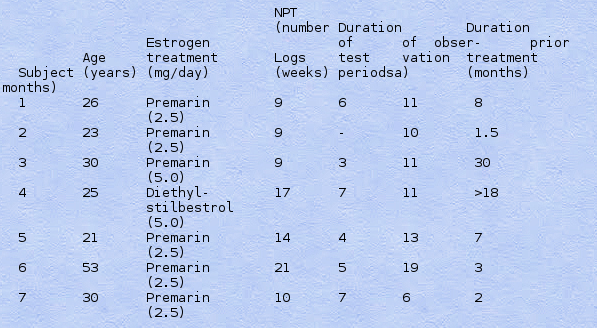
Table I. Description of Subjects' Treatments and Observation Periods, the Total Duration of Observation for All Different Measures, as Well as Time Elapsed from First to Last Observation and Durations of Estrogen Treatment before Onset of Observations
Throughout the experimental period, and at other times (see Table I), subjects kept daily logs and agreed to record therein all sexual acts: coitus, masturbation, petting, orgasms, spontaneous erections (those not generated in sex acts), and sexual feelings, by frequency per day. Logs were brought to the laboratory each week, at which time a brief interview was conducted and a 20-ml blood sample obtained. The blood was separated, frozen, and subsequently tested for testosterone level by radioimmunoassay (Frankel et al., 1975) and for sex-hormone-binding globulin (SHBG) by the filter paper method (Mickelson and Petra, 1974), from which estimated free testosterone levels were calculated (Wiest et al., 1978).
Physiological Observations
Quantitative assessments of nocturnal penile tumescence (NPT) for 2-5 nights were obtained using portable home monitors. Recordings that were technically questionable (e.g., due to strain gauge breakage) were eliminated during blind scoring of the data. The NPT data were evaluated in terms of (a) percentage of sleep time spent within 80% of the maximum magnitude (MM) of increased penile circumference per erectile episode multiplied by MM for that episode totalled over the night (% T.MM) and (b) percentage of sleep time during which MM was greater than 1.5 cm (% T > 1.5)
Erectile responses to sexual stimuli were measured by subjects observing erotic videotapes (or sexually neutral television programs) and generating sexual fantasies ("imagine the most sexually arousing experience possible"), all in complete privacy. The sequence of testing was neutral / fantasy / neutral / film / neutral / film / neutral / film / neutral / fantasy / neutral; each episode lasted 4 minutes. The data were analyzed to generate two amplitude x duration measures (T.MM and T > 1.5), similar to those derived for the NPT data.
Experimental Protocol and Analysis
Subjects volunteered to spend 2 months in a double-blind crossover experiment at a time of their choice within the longer series of clinical observations. One month on the subject's usual oral estrogen treatment was to be alternated with one month of receiving placebo. Daily log data were collected throughout the experimental period. Information on periods of data collection before and after the experimental period are presented in Table I, along with other details. Blood samples were taken two to four times times during the experimental period and also during the other observation periods. The decrease in testosterone level, due to negative feedback inhibition of gonadotropin by estrogen (Santen, 1975) or its absence during placebo administration, provided a weekly check on patient compliance.
RESULTS
Daily Logs
Daily log data collected during the experimental treatments were scored and expressed as weekly frequencies of spontaneous erections, sex acts (coitus was never reported, so these were always masturbatory), orgasms, and sexual feelings. Since sex acts were almost always orgasmic, the data on orgasm frequency were almost identical with those dealing with sexual acts. Placebo vs. estrogen data were compared statistically using the paired t test. There were no significant differences between these two conditions on any measure.
Examination of the data on erections, sexual acts, and orgasms plotted across time, however, revealed clear-cut changes appearing approximately 2 weeks following a change in treatment. Accordingly, the data were reanalyzed in terms of mean weekly responses during the 3rd and 4th weeks after the onset of a change in treatment. These 2-week periods were the last 2 weeks of each treatment; in the two cases where the placebo was administered for less than 4 weeks, the "placebo treatment periods" actually consisted of the first 2 weeks of Premarin treatment in subject 1 and 1 week of placebo plus 1 week of Premarin in subject 5. Figure 1 shows data on sexual acts and spontaneous erections arranged in this fashion. In all subjects, estrogen had the effect of decreasing the behavior, except for subjects 4 and 5, whose frequency of sex acts remained at 0 and 1 per week, respectively, during both treatment conditions. Spontaneous erection, sex act, and orgasm frequencies were significantly lower on estrogen than placebo (t = 3.75, p < 0.005, and t = 2.77, p < 0.025, 1-tailed, for erections and acts respectively).
Analysis of the data on frequency of sexual feelings by either of the two methods used above revealed no significant differences between conditions, in part because subjects 2, 3, and 6 reported no sexual feelings at all during the blind experiment. The subjects were requested to state their guesses as to which treatment was which. By 2 weeks after the onset of blind treatments, all had guessed correctly the identity of the treatment and attributed this to noticing the return of spontaneous erections while on placebo.
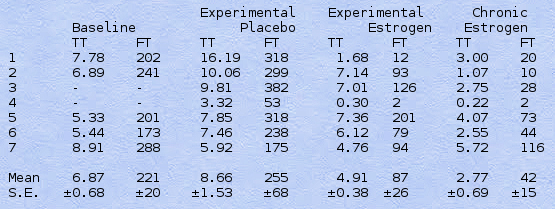
Table II. Mean Total (TT) and Free (FT) Plasma Testosterone Levels before Estrogen Treatment Was Initiated (Baseline); during the 3rd and 4th Weeks after Change of Treatment in the Experimental Period (Estrogen and Placebo); and for Periods of Over 2 Months of Estrogen Treatment (Chronic Estrogen). Mean values in ng/ml (TT) and pg/ml (FT) are shown. All baseline values are means of 3 samples, and chronic estrogen of 3 or 4. Experimental values are means of 2 samples or single ones (subjects 3, 6, 7).
Hormonal Data
Mean total and free testosterone levels are shown in Table II for the different phases of the study: before onset of estrogen treatment (baseline), during the experimental period, and during long-term estrogen treatment. Placebo and estrogen treatment periods were as defined in the previous section. During the baseline observations, total testosterone levels were well within the normal range (3-11 ng/ml). During placebo treatment, total and free testosterone levels were above baseline values in 4 of the 5 subjects with baseline data. Particularly high total and/or free levels were present in subjects 1 and 2. In all 7 subjects, the levels were lower during estrogen treatment than placebo, though only in subjects 1 and 4 did they drop to the hypogonadal range. Even after long-term treatment, subjects 5 and 7 had total testosterone values clearly within the normal range. In all cases, however, the percentage of decrease from placebo to estrogen conditions was considerably greater for free testosterone than for total testosterone. Thus, with estrogen treatment total testosterone dropped to an average of 60 +/- 13% (S.E.) of the placebo level, while free testosterone dropped to 23 +/- 9%. Subject 5 showed the least change in total or free testosterone levels with estrogen treatment; independent evidence confirmed inconsistent pill-taking in this case. In all cases, testosterone levels returned to the normal range of values for men by the third week after onset of placebo treatment, with the DES-treated subject showing the lowest value.
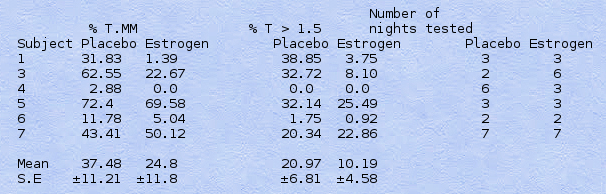
Table III. Nocturnal Penile Tumescence Data during Blind Experimental Period
Nocturnal Penile Tumescence
Data on NPT (see Table III) were available for only six of the seven subjects, since subject 2 would not use the NPT monitor. In addition, two subjects had extremely low NPT scores. Subject 4 showed a virtual absence of nocturnal erections, and this subject (receiving 5 mg DES) also had the lowest testosterone values of the group. Table III shows mean NPT results during placebo and estrogen conditions for all subjects. In light of considerable variability in the data, it was not surprising that there was no significant difference between the two experimental conditions, using the paired t test on either NPT variable (p > 0.05, 1-tailed). However, the trend was clearly toward a decline in NPT measures with estrogen; robust decreases were found in three subjects. This was further investigated by examining NPT-testosterone relationships both outside and within the experimental period. Free and total testosterone mean values for all subjects in all conditions were plotted against the concurrently collected mean NPT data. NPT data were obtained for two to five nights during 1-week periods, with blood sampling on days one and eight; the two testosterone values were averaged to generate a mean testosterone value used in the analysis.
Figure 2 shows a scattergram of the individual data on NPT vs. free testosterone from each period of observation, and Table IV presents mean coefficients of correlation for each individual subject. It is clear that NPT is positively related to free testosterone levels (though the scattergram was less impressive for total testosterone). Individual correlation coefficients were high and positive in all subjects except subject 7. The slopes of the regression lines (hormone vs. NPT) were calculated for each subject, and the significance of the slope was calculated by single-sample t test and found to show significance for total and free testosterone.
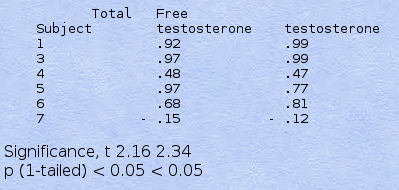
Table IV. Individual Correlation Coefficients for the Relationship between NPT (% T.MM) and Total or Free Testosterone over the Various Conditions and Significance of the Slopes of the Regression Lines
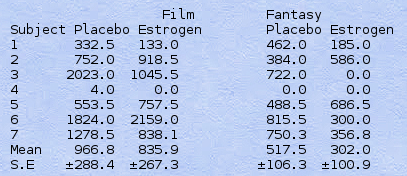
Table V. Psychophysiological Data: Erectile Responses to Film and fantasy (Amplitude x Duration, T.MM) during Experimental Period
A similar analysis was done for self-reported data on spontaneous erections and sexual acts. The daily log data were analyzed in blocks of six days, three each before and after the testosterone determination to which they were being related. Statistical significance was obtained (p < 0.05, t = 2.04, 1- tailed) for the relationship between free (but not total) testosterone and spontaneous erections, but not for the relationships between total or free testosterone and sexual acts.
Psychophysiological Data
Table V shows the psychophysiological responses to erotic film and fantasy. Three subjects showed an increased response to film with estrogen; three others decreased. Four of the seven subjects showed a marked decrease in response to fantasy during estrogen treatment, and two a less marked decrease. One subject (4) showed essentially no response to either stimulus. This was the same subject (receiving DES) who was only barely responsive on the NPT test. A paired t test revealed no significant differences between placebo and estrogen for the film or fantasy tests (p > 0.05, 1-tailed).
DISCUSSION
This report indicates that estrogen administration to genetic male transsexuals with intact testes reduces sexual activity, orgasms, and spontaneous erections. Changes in these measures of sexuality followed changes in hormone treatment by approximately 2 weeks. Objective assessment of spontaneous erectile capacity was obtained from periods of all-night recordings of nocturnal erections and the quantitative analysis of the resulting data. The relationship between free testosterone vs. NPT and selfreported spontaneous erection data following observations made in a variety of hormonal situations revealed a positive relationship between these variables. In each of these situations except the experimental period, hormone levels had reached steady-state conditions.
Though erectile responses to erotic film and fantasy were not found to be significantly affected by estrogen, the data are too variable to allow for reliable negative conclusions from such a small sample. However, Bancroft et al. (1974) found no reduction in film-induced erections in sex offenders whose testosterone levels were suppressed by estrogen.
The data suggest that spontaneous (waking or nocturnal) erections and sexual activity are testosterone-dependent, but this does not necessarily reflect an overall testosterone dependence of the intrinsic erectile mechanism. Similar (and firmer) conclusions have arisen from the study of testosterone treatment in hypogonadal men. Thus, Kwan et al. (1983) reported that sexual activity and NPT responses in these subjects were clearly testosterone-dependent, but not their responses to film and fantasy, while Bancroft and Wu (1983) found that testosterone affected the film but not the fantasy response. The additional conclusion from our work and that of others (Skakkebaek et al. 1980; Luisi and Franchi, 1980; Bancroft, 1980) that testosterone also independently stimulates libido was not reflected in the present results on frequency of sexual feelings. However, male-to-female transsexuals may commonly report a lack of sexual feelings before gender surgery is performed, for reasons unrelated to libido status.
Free testosterone was more suppressed by the estrogen treatment than total testosterone, as a result of elevated SHBG levels and consequent binding of testosterone. Because severe decreases in sexual function occurred when free testosterone level was reduced, but total testosterone was not below the normal range, and because free but not total testosterone was related to spontaneous erections, we tentatively conclude that the free fraction is responsible for effects on sexuality. This is consistent with our previous finding that the sexual decline in aging men is slightly but significantly correlated with low free but not total testosterone level (Davidson et al. 1983). The data provide no indication of a stimulatory effect of estrogen on any of the aspects of sexual function studied. However, it should be noted that the animal data showing positive effects of estrogen on sexual behavior are mostly limited to motivational (i.e., libido) measures (Beyer, 1979; Davidson, 1969) and do not include reflex erectile response (Gray et al. 1980; Hart, 1979). Finally, the possibility that estrogen inhibits sexual function directly, rather than via androgen suppression, cannot yet be excluded.
ACKNOWLEDGMENTS
We gratefully acknowledge the help of Drs. Donald Laub and Norman Fisk and thank Brenda Siddall for excellent technical assistance. Special thanks are due to Dr. Jeanette Chen, who began the work that led to this study. The antiserum for the testosterone assay was kindly supplied by Dr. B. Caldwell.
REFERENCES
- Bancroft, J. (1980). Endocrinology of sexual function. Clin. Obstet. Gynaecol. 7: 253-281.
- Bancroft, J., and Wu (1983). Changes in erectile responsiveness during androgen replacement therapy. Arch. Sex Behav. 12: 59-66.
- Bancroft, J., Tennent, T. G., Loucas, K., and Cass, J. (1974). Control of deviant sexual behavior by drugs: Behavioral effects of estrogens and antiandrogens. Brit. J. Psychiat. 125: 310-315.
- Bever, C. (1979). Endocrine Control of Sexual Behavior. Raven Press New York.
- Davidson, J. M. (1969). Effects of estrogen on the sexual behavior of male rats. Endocrinology 84: 1365-1372.
- Davidson, J. M. (1977). Neuro-hormonal bases of male sexual behavior. In Greep, R. O. (ed.), Reproductive Physiology II (International Review of Physiology, Vol. 13), University Park Press, Baltimore, Maryland, pp. 225-254.
- Davidson, J. M., Gray, G. D., and Smith, E. R. (1978). Animal models in the endocrinology of reproductive behavior. In Alexander, N. (ed.), Animal Models for Research on Contraception and Fertility Harper & Row, Hagerstown, Maryland, pp. 61-81.
- Davidson, J. M., Camargo, and Smith (1979). Effects of androgen on sexual behavior in hypogonadal men. J. Clin. Endocrinol. Metab. 48: 955-958.
- Davidson, J. M., Kwan, M., and Greenleaf, W. J. (1982). Hormonal replacement and sexuality in men. In Bancroft, J. (ed.), Clinics in Endocrinology and Metabolism (Vol. 11) Saunders, London, pp. 599-624.
- Davidson, J. M., Chen, J. J., Crapo, L., Gray, G. D., Greenleaf, W. J., and Catania, J. A. (1983). Hormonal changes and sexual function in aging men. J. Clin. Endocrinol. Metab. 57: 71-77.
- Davidson, J. M., Chen, J. J., Crapo, L., Gray, G. D., Greenleaf, W. J., and Catania, J. A. (1983). Hormonal changes and sexual function in aging men. J. Clin. Endocrinol. Metab. 57: 71-77.
- Frankel, A. I., Mock, E. J., Wright, W. N., and Kamel, F. (1975). Characterization and validation of a radioimmunoassay for plasma testosterone in the male rat. Steroids 25: 73.
- Gray, G. D., Smith, E. R., and Davidson, J. M. (1980). Hormonal regulation of penile erection in castrated male rats. Physiol. Behav. 24: 463-468.
- Hart, B. L. (1979). Activation of sexual reflexes of male rats by dihydrotestosterone but not by estrogen. Physiol. Behav. 23: 107-109.
- Kwan, M., Greenleaf, W. J., Mann, J., Crapo, L., and Davidson, J. M. (1983). The nature of androgen action on male sexuality: A combined laboratory-self-report study on hypogonadal men. J. Clin. Endocrinol. Metab. 57: 557.
- Luisi, M., and Franchi, F. (1980). Double-blind group comparative study of testosterone undecanoate and mesterolone in hypogonadal male patients. J. Endocrinol. Invest. 3: 305-308.
- Mickelson, K. E., and Petra, P. H. (1974). A filter assay for the sex steroid binding protein (SEP) of human serum. FEBN Letters 44: 34.
- Santen, R. J. (1975). Is aromatization of testosterone to estradiol required for inhibition of LH secretion in man? J. Clin. Invest. 56: 1555- 1563.
- Skakkebaek, N. E., Bancroft, L., Davidson, D. W., and Warner, P. (1980). Androgen replacement with oral testosterone undecanoate in hypogonadal men: A double-blind controlled study. Clin. Endocrinol. 14: 49-61.
- Wiest, W. G., Paulson, J. D., Keller, D. W., and Warren, J. C. (1978). Free testosterone concentration in serum: A method for determination. Amer. J. Obstet. Gynecol. 130: 321.
Comments
comments powered by Disqus