by H. Asscheman, L.J.G. Gooren, and P.L.E. Eklund
Sex steroid treatment is associated with side effects. The number of deaths and morbidity cases in 425 transsexual patients treated with cross- gender hormones were evaluated retrospectively and compared with the expected number in a similar reference group of the population. The number of deaths in male-to-female transsexuals was five times the number expected, due to increased numbers of suicide and death of unknown cause. Combined treatment with estrogen and cyproterone acetate in 303 male-to-female transsexuals was associated with a 45-fold increase of thromboembolic events, hyperprolactinemia (400-fold), depressive mood changes (15-fold), and transient elevation of liver enzymes. Androgen treatment in 122 female-to- male transsexuals was associated with weight increase >10% (17.2%) and acne (12.3%). In both groups persistent liver enzyme abnormalities could be attributed to other causes than sex steroids (hepatitis B and alcohol abuse). Much of the morbidity was minor and reversible with appropriate treatment or temporary discontinuation of hormone treatment. Thus, the dilemma of prescribing cross gender hormones in view of the needs of these patients is not resolved. Explanation of possible side effects and careful clinical judgment remain the cornerstone of the clinical decision to prescribe cross- gender hormones. Furthermore, follow up of this relatively young population to disclose long-term side effects and to elucidate the association of sex steroids with coronary heart disease, as well as efforts to reduce the risk of thromboembolic events, are required. Transsexual seek to adapt their bodies to the opposite biologic sex to which they perceive themselves belonging. Hormonal treatment plays an important role in this process.1 Ideally, the given hormone treatment should suppress the secondary sex characteristics of the original sex, as well as induce those of the opposite sex to the fullest extent and in the shortest possible period of time. Therefore, there is an inclination to maximize hormone dosage, which may involve health hazards.
Side effects of sex steroid therapy in more conventional categories of patients have been extensively reported: oral contraceptives in women of reproductive age,2,3 estrogen substitution in postmenopausal women, 4-6 estrogen treatment of prostatic carcinoma,7 and androgen replacement in men.8 Reports on side effects of cross-gender hormone treatment in transsexuals have been few. One study evaluating the efficacy of estrogens and androgens in 90 transsexual patients documented only liver enzyme abnormalities and mild elevations of cholesterol and triglyceride levels.9 Case reports have described pulmonary embolism,10 cerebral thrombosis,11 myocardial infarction,12 prostatic metaplasia,13 and breast cancer14,15 in estrogen-treated male-to-female transsexuals and recurrent myocardial infarction in a female-to-male transsexual treated with androgens.16 We report here a retrospective investigation into the side effects of cross gender hormone administration in 425 transsexuals treated between 1972 and 1986.
Materials and Methods
The files of all patients seen in our outpatient department between 1972 and 1986 were reviewed Diagnosis of transsexualism followed the Standards of Care of the Harry Benjamin Gender Dysphoria Association.17 There were files of 558 patients with a problem of gender dysphoria, of whom 425 were included in this study. Reasons for exclusion of the others were as follows: no hormonal treatment (n = 71), only antiandrogen treatment (n = 6), failure to comply with our follow-up schedule (n = 15), no data available (n = 10), and treatment either not yet started or for less than six months (n = 31). The population included in this analysis comprised 303 male-to-female and 122 female-to-male transsexuals. Our standard hormone treatment regimen in male-to-female transsexuals consisted of 100 mg cyproterone acetate and 100 mg ethinylestradiol/ day orally (n = 258). Until 1980 diethylstilbestrol (5 to 15 mg/d) was prescribed for a few patients. Some patients insisted on parenteral estrogen therapy. They procured the estrogens outside our clinic and these were self-administered in a dose of 200 to 800 mg estradiol-17-undecanoate/month in (n = 45). Recommended dose of parenteral estrogens in postmenopausal women is 20 to 100 mg/m.
In female-to-male patients long-acting testosterone esters 250 mg im every 2 weeks (n = 69) or testosterone undecanoate 120 to 160 mg/d orally (n = 19), or both, but not simultaneously (n = 34), were prescribed. If menstrual activity did not cease within three months after start of the hormone administration, an oral progestin, lynestrol 5 mg/d, was added (n = 3).
The patients' ages at the start of hormone treatment ranged from 16 to 67 years (median 32 years) in male-to-female patients and 16-54 years (median 25.4 years) in female-to-male patients. The duration of hormone therapy ranged from six months to more than 13 years (median 4.4 years in male-to-female and 3.6 years in female-to-male patients). In the first two years of treatment, patients were seen every three months and every six to nine months thereafter. At each visit physical changes and complaints were recorded and a general physical examination was carried out. At least once, and generally twice a year, blood samples were drawn for determination of liver enzymes, prolactin levels, and, if indicated, other laboratory parameters. Diagnosis of venous thrombosis or pulmonary embolism was confirmed by phlebography or lung scintigraphy. Twelve transsexuals died during this follow-up period. The cause of death was determined either by autopsy report or from information given by the patients's general practioner. The expected mortality and morbidity of a comparable group of subjects were calculated from published reports on the incidence of mortality and similar morbidity in the general population aged 15 to 64 years. The expected number of cases was adjusted for age (15-24, 25-39, and 40-64 years of age) and sex,18 as well as for the period of time the study population was on cross-gender hormone treatment.
Results
The observed and the expected mortalities in male to-female transsexuals treated with estrogens and cyproterone acetate are shown in Table 1. The total number of deaths in the treatment group represented a 2.5- to 9-fold increase over the general population. The increased mortality was in particular due to an increase of suicides and of deaths by unknown cause. There may have been a connection to sex steroid treatment in the three deaths of unknown cause, although autopsy in two of these cases failed to demonstrate a hormone-related cause. All other causes of death were within the 95% confidence intervals of the expected mortality. As can be expected in a relatively young age group, six of the 12 deaths (95% Cl, 2.5-9.5) were not from natural causes.
A summary of all morbidity encountered and, if data were available, the number of expected cases in the general population, aged 15 to 64 years, is shown in Table 2 (male-to-female transsexuals) and Table 3 (female-to-male transsexuals).
A clinically serious side effect of estrogen treatment (45-fold increase) was venous thrombosis of the legs (n = 13) and/or pulmonary embolism (n = 6). Of the total male to-female transsexual group this occurred in 19 (6.3%) patients, yet was expected in only one patient. In the 235 male-to-female transsexuals who underwent sex reassignment surgery 4 (1.7%) were diagnosed postoperatively with venous thrombosis and/or pulmonary embolism. This low postoperative incidence in comparison to the total incidence of thromboembolic events in our patients is probably due to our policy of discontinuing all hormone treatment at least 4 weeks before surgery. If thrombosis attributed to estrogen occurred, this happened most often during the first year of treatment (ten of 13 cases). Furthermore, there was a strong association between age and the incidence of thrombosis: five of 237 (2.1%) patients under 40 years of age v eight of 66 (12%) patients over 40 years (P
The two cases of myocardial infarction occurred, after several years of hormone treatment, in one 45- and in one 50-year-old male- to -female transsexual. Both had a strong positive family history of heart disease and one also smoked 50 cigarettes a day.
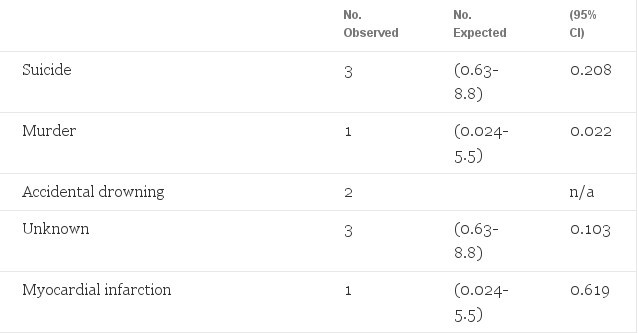
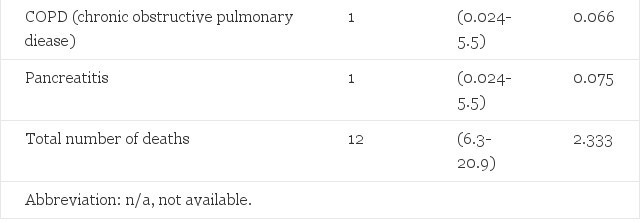
Table 1. Observed Mortality in 303 Male-to-Female Transsexuals Treated With Estrogens and Cyproterone Acetate, and Expected Mortality in the General Male Population 15 to 64 Years of Age (Adjusted for Age 15 to 24, 25 to 39, end 40 to 64 Years)
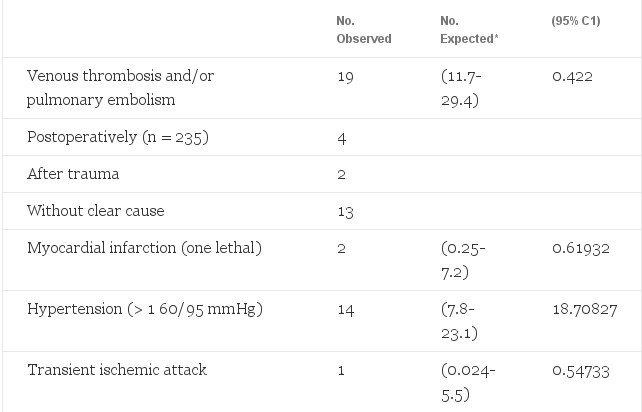
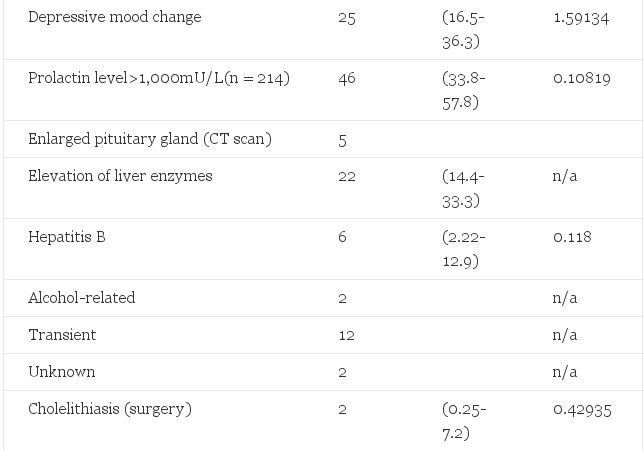
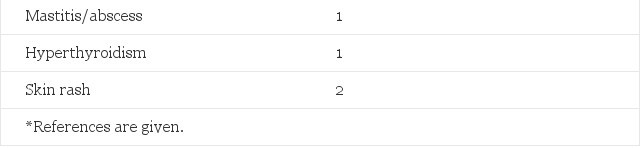
Table 2. Observed Morbidity in 303 Male-to-Female Transsexuals Treated With Estrogens and Cyproterone Acetate and Expected Number of Cases in the General Male Population 15 to 64 Years of Age (Adjusted for Age 15 to 24, 25 to 39, and 40 to 64 Years)
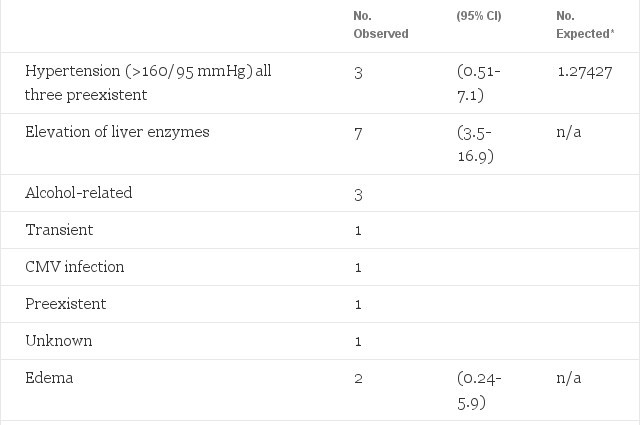
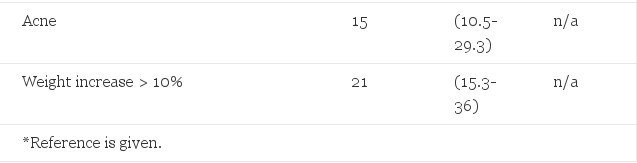
Table 3. Observed Morbidity in 122 Female-to-Male Transsexuals Treated With Long-acting Testosterone Esters or Testosterone Undecanoete, and Expected Morbidity (Adjusted for Age)
For four patients with preexistent hypertension, kept adequately under control through medication (160/95 mmHg) during sex steroid treatment. With appropriate antihypertensive treatment we were able to manage this without discontinuing the steroids.
In the first six months of hormone treatment depressive mood changes were reported spontaneously by 25 (8.3%) male-to-female patients. We did not specifically inquire into depressive symptoms nor was psychometric testing done of these patients. However, before the start of treatment all patients were informed of the possible effects hormones could have on their mood.
Serum prolactin levels increased in all estrogen-treated patients. In 46 (21.4%) patients serum prolactin levels rose to more than 1,000 mU/L (upper limit for males 300 mU/L), whereas only 0.108 of 214 patients were expected according to published prevalence rates.19 After reduction of the estrogen dosage serum prolactin levels fell to only slightly elevated values in 23 patients. CT scanning of the pituitary with contrast enhancement was performed in 15 of the 23 remaining patients (eight were either lost for follow-up or they refused radiologic evaluation). In five of these 15 patients pituitary enlargement was documented.20
In female-to-male patients androgen treatment deepened the voice, increased body hair growth, and induced beard growth to a varying extent. Shaving became necessary in >90% of them after one year of androgen treatment and some grew a full beard. Severe acne was observed in 15 (12.3%) subjects. Topical treatment for the acne was moderately effective in most patients. All 21 patients (17.2%) who gained more than 10% body weight were already obese before the start of testosterone administration. Minor weight increase (I to 3 kg) was common in both estrogen- and androgen-treated patients, but minor weight loss was also regularly documented.
Discussion
This study is a retrospective and not a case-control study. Therefore, no definitive conclusions about the relative risks of cross-gender hormone treatment can be drawn. The conclusion of this study could have been more firm if a control group had been included. It did not appear feasible to match our group, which was heterogeneous in age and duration of hormone treatment, with a control group. In the latter group there should have been a similar follow-up with regular clinic visits and laboratory tests. To compensate for this drawback, we compared our findings with the expected number of deaths and morbidity calculated from health statics, adjusted for age and sex, of the general population. This approach still poses a problem because our group was selected in two ways: (1) in our clinical population serious preexistent morbidity was excluded (eg, serious cardiovascular disease), and (2) the patients were treated on the basis of their transsexualism, which might be associated with a greater proneness to some pathologies (eg, suicide). How ever, these comparisons are the best available for clinical evaluation of cross-gender hormone treatment. Compliance with the prescribed hormones was obvious from the induced physical changes and was not monitored in this study.
The increased mortality in male-to-female transsexuals compared with the expected mortality can be partly explained by the increased suicide rate. Many patients (14%, unpublished observations of our group) attempted suicide before the start of sex reassignment treatment. This increased suicide rate confirms the notion that gender reassignment is not fully effective in relieving co-existent morbidity in cases of gender dysphoria. The increased number of deaths by unknown cause does raise concern. Two of these three deaths might have been suicide, according to friends and relatives. The autopsy of one did not include toxicologic screening; thus, the assumption of suicide cannot be refuted. More extensive follow-up will be necessary to evaluate the risk of death by unknown cause.
It is evident from our results that the incidence rates of venous thrombosis and/or pulmonary embolism (45-fold), hyperprolactinemia (400-fold), depressive mood changes (15-fold), and transient elevation of liver enzymes were increased with estrogen and/or cyproterone acetate use, whereas weight increase and acne were associated with androgen use.
In accordance with previous studies of hyperlipemic men treated with estrogens,2l our study shows an increased incidence of thrombosis in estrogen-treated male-to-female transsexuals. The observed incidence in our study of 1,140 of 100,000 exposure years is similar to the incidence of 1,100 of 100,000 exposure years calculated from the data of the Coronary Drug Project Research Group.21 In comparison, the incidence of thromboembolic disease in oral contraceptive users is 300 of 100,000 exposure years and in healthy men estimated at 30 of 100,000 exposure years.2 The magnitude of this increased incidence rate of thromboembolic events is worrisome and should be a stimulant for research for less thrombogenic hormone schedules. Either reduction of the estrogen dosage to the lowest possible effective dose, similar to what has been shown with oral contraceptives, or another way of administering, eg, percutaneous estradiol with less hepatic effects, might be effective ways of reducing this risk. We also found advanced age (>40 years) to be a factor affecting the incidence of documented venous thrombosis and pulmonary embolism in male to-female transsexuals using estrogens. The latter results have also been reported in women using oral contraceptives.3
The connection between sex hormone levels, coronary heart disease, and coronary risk factors is complex.22 Epidemiologic studies suggest that changes in blood lipid levels similar to those that occur during estrogen treatment reduce the risk of coronary heart disease.23 Yet, it has been found that estrogens did not prevent recurrent episodes of clinical coronary heart disease in hyperlipemic men with previous myocardial infarction.21 Furthermore, in prostatic cancer patients, treatment with estrogens has been associated with an increased incidence of cardiovascular events24 and mortality.25 Our follow-up study of relatively young and healthy gender dysphoric males has still been too short to confirm any association.
The effect of androgens on blood lipids (increasing total cholesterol and decreasing HDL-cholesterol26 has also been found in women treated with testosterone cypionate (100 to 800 mg/mo im) for transsexualism.9 In this cross-sectional study these authors reported an increase of the total cholesterol levels in the low (100 to 300 mg/mo) and high dosage (800 mg/mo) regimen (11 observations) and no increase in the medium dosage of 400 mg (30 observations). This last dosage is comparable to the dosage we used. Our limited number of prospective observations, as yet insufficient for statistical analysis, shows a highly variable response. Theoretically, the increased serum total cholesterol and decreased HDL-cholesterol level could increase the risk of coronary heart disease. To our knowledge, only one case report supports this assumption, although pretreatment serum lipid levels in this case were not available.16 The relatively young age and the follow-up period of median 3.6 years in our study preclude a definite conclusion.
The number of cases of hypertension observed is slightly less than expected. This observation is in contrast with the reported effect of oral contraceptives on blood pressure in women.2 Meyer et al9 also failed to find changes of arterial blood pressure in estrogen-treated male-to-female transsexuals. This discrepancy with the findings in oral contraceptive users may be explained by the way blood pressure readings were made. In their study and in the present study blood pressure was measured during clinic visits in patients who often came for the first time to a gender clinic. Blood pressure readings in this setting tend to be higher because of the nervous stress associated with new experiences. At follow-up visits the blood pressure decreases and can mask the increase caused by estrogens. Another reason we did not observe more cases of hypertension in the study group may be the relatively high prevalence of hypertension in the Dutch male population aged 15 to 49 years.27 This could obscure an increased incidence associated with estrogen and/or cyproterone acetate. More recent studies on blood pressure in women taking estrogens as replacement treatment also contradict the general assumption that estrogen treatment is associated with an increase in blood pressure. Actually, the majority of women in these studies experienced a decline in blood pressure.28,29
Temporary mood changes were spontaneously reported by a considerable number of male-to-female transsexual patients in the first six months of treatment but subsequently waned. In particular, cyproterone acetate has been reported to be associated with a lack of energy and depressive mood changes.30 The reason for these mental changes may not only be the hormone effect but also the psychologic pressure of living in the new gender role. Because depressive mood changes were found predominantly in the first six months of the hormone treatment, it is improbable that there has been an association with the three observed suicides: they occurred after 1.5, 4, and 8 years of hormone treatment.
If liver enzyme abnormalities were observed, patients were examined by hepatitis serology, ultrasonography of the liver, and follow-up of liver enzyme tests, in particular, if applicable after discontinuation of alcohol abuse. Most persistent liver enzyme abnormalities could be attributed to hepatitis B and alcohol abuse. No evidence of liver tumors, such as peliosis hepatis or hepatic adenoma, was observed. Transient liver enzyme abnormalities, similar to those found in the present study, have also been observed in estrogen-treated prostatic cancer patients but did not result in liver damage.31
The other side effects of cross-gender hormone treatment were minor. Many side effects were reversible with appropriate therapy or temporary discontinuation of hormones. The occurrence of serious side effects (eg, the prevalence of thromboembolic disease of 6.3%) was, however, not rare. In view of the needs of transsexuals these side effects present a difficult dilemma in hormonal gender reassignment. At present no firm guidelines can be given. The cornerstone of the decision to prescribe cross-gender hormones remains with the explanation of the possible side effects to the patients and careful clinical judgment. Further follow-up of this relatively young population to disclose long-term side effects and to elucidate the association of sex steroids with coronary heart disease, as well as efforts to reduce the risk of thromboembolic events, are required.
Acknowledgements
The authors thank Jos Megens for his invaluable administrative and organizational contributions to this study, Elisabeth Savage, MA, and Dr Keith Courtney for their editorial assistance, and Dr Jos Nauta for his statistical advice.
© Asscheman H, Gooren LJ, Eklund PL.(1989) Metabolism. Sep;38(9):869-73.
References
- Hamburger C: Endocrine treatment of male and female transsexualism, in Green R, Money J (eds): Transsexualism and Sex Reassignment. Baltimore, John Hopkins University, 1969, pp 291- 307
- Stadel BV: Oral contraceptives and cardiovascular disease. N Engl J Med 305:612-618, 672-677,1981
- Royal College of General Practitioners Oral Contraception Study: Mortality among oral-contraceptive users. Lancet 2:727-731, 1977
- Bush TL, Cowan LD, Barrett-Connor E, et al: Estrogen use and all-cause mortality. JAMA 249:903-906,1983
- Stampfer MJ, Willett WC, Colditz GA, et al: A prospective study of postmenopausal estrogen therapy and coronary heart disease. N Engl J Med 313:1044-1049,1985
- Shapiro S, Kelly JP, Rosenberg L, et al: Risk of localized and widespread endometrial cancer in relation to recent and discontinued use of conjugated estrogens. N Engl J Med 313:969-972,1985
- Veterans Administration Co-operative Urological Research Group: Treatment and survival of patients with cancer of the prostate. Surg Gynecol Obstet 124:1011-1017, 1967
- Wilson JD, Grimn JE: The use and mis -u s e of androgens. Metabolism 29:1278-1295, 1980
- Meyer WJ, Webb A, Stuart CA, et al: Physical and hormonal evaluation of transsexual patients. A longitudinal study. Arch Sex Behav 15:121-138,1986
- Lehrman KL: Pulmonary embolism in a transsexual man taking diethylstilbestrol. JAMA 235:532-533,1976
- de Marinis M, Arnett EN: Cerebrovascular occlusion in a transsexual man taking mestranol. Arch Intern Med 138:1732-1733, 1978
- Fortin CJ, Klein T, Messmore HL, et al: Myocardial infarction and severe thromboembolic complications as seen in an estrogen dependent transsexual. Arch Intern Med 144:1082-1083,1984
- Goodwin GE, Commings RH: Squamous metaplasia of the verumontaniem with obstruction due to hypertrophy: Long-term effects of estrogen on the prostate in an aging male to female transsexual. J Urol 131:553-554,1984
- Symmers WSC: Carcinoma of the breast in transsexual individuals after surgical and hormonal interference with primary and secondary sex characteristics. Br Med J 2:83-85,1968
- Pritchard TJ, Pankowsky DA, Crowe JP, et al: Breast cancer in a male- to -female transsexual. JAMA 259:2278-2280,1988
- Ffrench-Constant CK, Spengel FA, Thompson GR: Hyperlipidaemia and premature coronary artery disease associated with sex- change in a female. Postgrad Med 61:61-63, 1985
- Walker P, Berger J, Green R, et al: Standards of care. The hormonal and surgical sex reassignment of gender dysphoric per sons. Arch Sex Behav 14:79-90,1985
- Netherlands Central Bureau of Statistics: Statistisch Zak boek (Pocket Yearbook). The Hague, Staatuitgeverij, 1987
- Miyai K, Ichihara K, Kondo K, et al: Asymptomatic hyperpro lactinacmia and prolactinoma in the general population. Mass screening by paired assays of serum prolactin. Clin Endocrinol (Oxf) 25:549-554, 1986
- Asscheman H, Gooren LJG, Assies J, et al: Prolactin levels and pituitary enlargement in hormone-treated male-to-female transsexuals. Clin Endocrinol (Oxf) 28:583-588,1988
- Coronary Drug Project Research Group: Findings leading to discontinuation of the 2.5 mg/day estrogen group. JAMA 226: 652- 657, 1973
- Sex, hormones, and atherosclerosis. Lancet 2:552-553, 1986 (editorial)
- Gordon T, Castelli WB, Hjortland MD, et al: High density lipoprotein as a protective factor against coronary heart disease. The Framingham study. Am J Med 62:707-714, 1977
- Henriksson P, Edhag O: Orchidectomy versus oestrogen for prostatic cancer: Cardiovascular effects. Br Med J 293:413-415, 1986
- Glashan RW, Robinson MRG: Cardiovascular complications in the treatment of prostatic carcinoma. Br J Urol 53:624-627,1981
- Kirkland RT, Keenan BS, Probstfield JL, et al: Decrease in plasma high-density cholesterol levels at puberty in boys with delayed adolescence. Correlation with plasma testosterone levels. JAMA 257:502-507, 1987
- Van Loo JML, Drenthen AJM, Peer PGM, et al: Prevalentie, opsporing en behandeling van hypertensie in Lelystad (1982-1984); is "de regel van de helften" nog steeds van toepassing? Ned Tijdschr Geneeskd 131:624-627, 1987
- Wren BG, Brown LBH, Routledge DA: Differential clinical response to oestrogens after menopause. Med J Aust 2:329-332, 1982
- Lind T, Cameron EC, Hunter WM, et al: A prospective, controlled trial of six forms of hormone replacement therapy given to postmenopausal women. Br J Obstet Gynaecol 86:1-29,1979 (suppl 3)
- Braendle W, Boesse H, Breckwoldt M, et al: Wirkung und Nebenwirkung der Cyproteronacetatbehandlung. Arch Gynak 261: 335-345, 1974
- Kontturi M, Sotaniemi E: Effect of oestrogen on liver function of prostatic cancer patients. Br Med J 4:204-205,1969
- Hoogendoorn D: Atherosclerotic diseases of the heart and in the statistics of causes of deaths. Ned Tijdschr Geneeskd 129: 1827- 1833, 1985
- Hoogendoorn D: Atherosclerotic diseases of the brain as seen in hospitals and in the national statistics of causes of death; the cerebrovascular accident (CVA). Ned Tijdschr Geneeskd 129: 1883-1887, 1985
- Oliemans AP: Morbidity in the general practice. Doctoral thesis, Leiden, Stenfert Kroese, 1969
- Hoogendoorn D: Remarkable changes of the epidemiological pattern of cholithiasis, cholecystitis and gall-bladder cancer. Ned TijdschrGeneeskd
Comments
comments powered by Disqus