Hormonal therapy for transsexual patients undergoing gender re-assignment suffers from a lack of knowledge about possible adverse effects. This article presents a description of some of the drugs we have found useful for management of these patients and gives an outline of our scheme for monitoring change.
The endocrinological management of transsexualism is generally not started until after assessment. The assessment determines the patient's diagnosis, commitment and their likely success with gender re-assignment. This is in line with the recommendations of the International Gender Dysphoria Association. It reflects the fact that some changes can be irreversible.
Many of the drugs involved will be familiar to doctors due to their use in other conditions. In fact, due to the relative rarity of transsexualism, none of the products has undergone clinical trials in this group of patients to the extent that a manufacturer could obtain a product licence. Therefore, it is important for practitioners to recognise that there are no manufacturer's product liabilities for use in transsexual patients. The absence of a product liability, however, should not prevent a doctor exercising professional judgement with respect to treatment.
The absence of large scale trials in this field, however, means that good scientific evidence on which to base such judgements is thin on the ground. Therapeutic decisions are based largely on data obtained in other groups of patients, intuitive assumptions ultimately, within patient therapeutic trials. This is not ideal, but it is unavoidable if this small group of individuals is to receive medically supervised treatment.
Without such treatment, some patients may experience severe, long term psychological effects and, without treatment, some will obtain less appropriate drugs (e.g., contraceptive pills from a relative). Given that doses in excess of those recommended for conventional purposes may be required to produce desired effects, it is obviously preferable to avoid this situation.
Treating male-to-female patients
Inducing and enhancing feminine characteristics and suppressing masculine characteristics are the objectives of pharmacological therapy in this group. (Table 1) It is not possible to alter totally all gender-specific traits by hormonal means. For example, postpubertal male voice pitch will not be affected by medication as the larynx will already be irreversibly changed. Speech therapy is indicated to modify this.
Oestrogens
- Conjugated equine oestrogens orally, 5-10mg daily
- Ethinyloestradiol orally, 10-50 micrograms daily
- Oestradiol implants, 100mg monthly
- Oestradiol transdermal patches, 50-150 micrograms twice-weekly
Androgen suppression
- Cyproterone acetate orally, 50-150mg daily
- Nafarelin nasal spray, 600 micrograms daily
- Goserelin implant, 3.6mg monthly
Table 1. Prescription for male-to-female gender reassignment
Oestrogens
The desired effect of oestrogens is development of a more feminine figure by accumulating subcutaneous body fat, particularly over the hips and breasts. Breast development may follow the same stages as normal female puberty with initial growth beneath the areola and eventual nipple enlargement and pigmentation. The degree and speed of such changes varies but they are probably dose-dependent. Other appropriate effects, such as retardation of the male pattern of balding and a more luxuriant growth of head hair may be due to increased levels of sex hormone-binding globulin (SHBG). Paradoxically, loss of scalp hair and hirsutism have, on rare occasions, been reported with conjugated oestrogens. Body and beard hair consistency may also be softened,but there is no direct effect on beard growth itself. Other measures are needed to control this.
The side effects of oestrogen therapy in transsexual patients may be inferred from experience with contraception and menopausal hormone replacement therapy (HRT). This is considerable doubt as to whether there is any increased risk of thromboembolic events with natural oestrogens, at least in the doses used for HRT. [1] Therefore we generally prefer natural oestrogen compounds for the higher doses needed for gender re-assignment. Similarly, non-oral administration is preferred to avoid passage of the drug through the liver and its possible conversion to less advantageous metabolites. [2] These considerations do not preclude the occasional, judicious use of orally administered synthetic oestrogens to some patients - usually those who commenced such treatment elsewhere.
It is known that oestrogens stimulate pituitary release of prolactin. [3] This may occur with exogenous intake of oestrogens or in pregnancy. The effect is not restricted to genetic females and the high oestrogen doses used to induce feminisation in genetic males may produce similar effects (see Figure 1) There may occasionally be radiological evidence of pituitary enlargement in such patients, although we have never encountered lateral visual field disturbances or other features of compression of the optic chiasma.
We previously used progestogens in the belief that they would complement oestrogenic effects on breast growth. However, we have become less convinced of the importance of this effect and wary of progestogenic side effects, including severe myalgia in some cases.
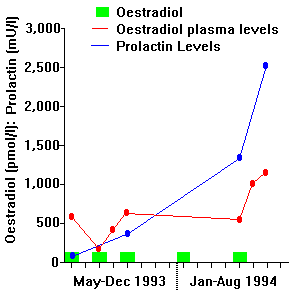
FIGURE 1 - Patient treated with periodic 100mg oestradiol implants. As plasma oestradiol levels rise in the normal premenopausal range, prolactin levels become abnormally elevated
Androgen Suppression
The desired effects with androgen suppression are a reduction in the rate of beard and body hair growth and further softening of the hair. The female distribution of pubic hair should also arise should also arise following androgen suppression. Spontaneous erections also occur less frequently. These effects can be achieved by suppressing androgen output as well as blocking the androgen receptors. The main drug used for these purposes is cyproterone acetate. This has been marketed for the treatment of male hypersexuality and prostatic cancer and has been combined with ethinyloestradiol for the treatment of female hirsutism and acne. Unfortunately, prolonged use at high doses is associated with an increased risk of potentially severe hepatoxicity. [4]
Increasingly, it is seen as more appropriate to use gonadotrophin-releasing hormone (GnRH) analogues for this purpose. These drugs produce initial stimulation followed by rapid, profound suppression of the hypothalamic-pituitary-gonadal axis. The result is a reduction in cyclical follicle-stimulating hormone output and, therefore, the absence of gonadal androgen production. The disadvantage of these drugs is that they cannot be given orally and are still relatively expensive. In some cases, it may be appropriate to bring forward surgical intervention if these compounds are prescribed. Side effects are relatively minor, but include nasal irritation, headaches and nervous response.
Monitoring During Treatment
A periodic review of the clinical progress with respect to the desired effects of these drugs is based principally on the patient's subjective impressions. Even semiquantitative assessments, such as frequency of shaving or electrolysis, are dependent upon accurate information from the user. Measuring of breast and hip size and assessing each stage of nipple development are more objective, but they distress and embarrass some patients. Clinical assessments are usually conducted on a three-monthly basis, as are blood assays of oestradiol and testosterone.
Such measurements are needed to exclude excessive oestrogen levels when the dosage needs to be increased to maintain a given response (tachyphylaxis). Where synthetic ethinyloestradiol is being used, it is important to appreciate that it will be undetectable by most assays for oestradiol. Requests for assay should specifically ask for ethinyloestradiol levels.
To minimise undesirable side-effects, blood pressure and weight should be recorded at each visit and, every three months, assays of liver function, lipid profiles and prolactin should be carried out. Baseline measurements should be taken before starting treatment. Assays may be repeated more often if indicated.
Treating female-to-male patients
Transsexuals who are undergoing female-to-male re-assignment must be made aware of the lack of the success in surgical construction of a functional phallus. For this reason, phalloplasty is usually not undertaken. The prescription of androgens, therefore, forms the major part of the gender re-assignment. However, menstrual suppression is often a higher priority for this group (Table 2)
Androgens
- Testosterone undecanoate orally, 120-160mg daily
- Testosterone intramuscular injection, 100-250mg alternate weeks
- Testosterone implants BP, 100-200 micrograms one to three times monthly
Menstrual suppression
- Nafarelin nasal spray, 600 micrograms daily
- Goserelin implant, 3.6mg monthly
Table 2. Prescription for female-to-male gender reassignment
Androgens
Hormone therapy will induce a deepening of the voice. Other desirable masculinising effects include coarsening of body hair, male pubic hair pattern, beard growth, muscle development and loss of subcutaneous fat.
If there is a familial predisposition to male pattern balding, this male also be expected to occur with androgen therapy. This particular masculinising effect is not always welcome and patients should be warned of its likelihood. The same is true of effects on mood, such as increased aggressiveness or libido.
Other side effects include hepatotoxicity [6], especially with orally administered 17-alkylated compounds, and increased risk of cardiovascular disease, including thromboembolism [7]. Awareness of such effects has been raised recently by the abuse of anabolic compounds by athletes. Similar high dose, prolonged use in transsexuals needs careful monitoring, even where compounds are regarded as being relatively free from problems (Figure 2).
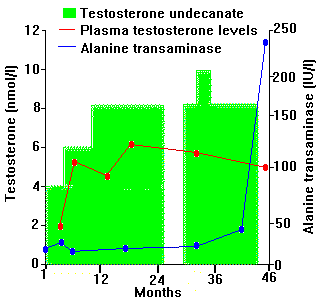
FIGURE 2 - During treatment with testosterone undecanoate, plasma testosterone levels do not rise to normal male levels but, after increasing doses, alanine transaminase becomes elevated
Menstrual Suppression
We found that most thorough relief from the inappropriate continuation of menstruation can be achieved with GnRH analogues, as discussed above. In these patients, it is usually preferable to start therapy in the first few days of a cycle.
Monitoring During Treatment
The monitoring of response to hormonal therapy in female-to-male transsexuals follows the same principals and pattern as for male-to-female gender re-assignment. Subjective measures of response include menstrual pattern, distribution of hair and voice change.
Pre- and Post-Operative Hormonal Therapy
There is some doubt about the association between natural oestrogens and thromboembolic phenomena, but it would seem wise to avoid any potential added risk of deep vein thrombosis in these patients undergoing relatively prolonged, major surgery in the perineal region. [8] We recommend that hormonal therapy is discontinued for one month before surgery. If therapy is to be continued post-operatively, it should not be recommenced until full immobilisation has been achieved. The need to continue therapy will depend upon the nature of the cosmetic surgery undertaken and individual needs. For example, in some cases, even where orchidectomy has been carried out, some patients request continued antiandrogen therapy because of persistent hair growth. This may be related to the duration of the growth phase of body hair after previous androgenic stimulation and, therefore, should only be a transient effect.
Similarly, genital reconstruction and breast implant insertion does not necessarily reduce the patient's perception of the need to continue oestrogen therapy to maintain feminisation. Such individual requirements are additional to the theoretical need to continue hormone therapy because of long term skeletal risks.
Although osteoporosis is recognised in hypogonadic men as well as women, it remains to be confirmed that bone loss is halted or reduced by prescription of sex steroids of the opposite gender. Where hormonal therapy continues after surgery, it should be with the lowest dose appropriate to the individual's symptoms and with benefit to continued monitoring.
GP Supervision
The role of the GP following the patient's referral for pharmacological management must not be underestimated, as the progress through gender re-assignment is often arduous for the patient. Up-to-date and ongoing information on the patient's mental and physical health and social status helps in assessing the patient's needs. An individual's response to sex steroids is often idiosyncratic and the review of medication takes this into account. Repeat prescription of the medication suggested by the clinic will usually have to be undertaken by the GP. Once gender re-assignment has been completed, a maintenance dose may be needed.
In spite of the difficulties encountered in the prescription of sex steroids, the transsexual's drive for re-assignment may lead him or her to procure and use these drugs without supervision. Clinical monitoring is ideally maintained once gender re-assignment has been completed, either through the clinic or the patient's GP. But after re-assignment, patient's may move area or change practices, often losing contact with the gender identity clinic.
Raising Awareness
Efforts in the treatment of these patients coincide with the principals expressed in the Health of the Nation, [9] relating to the reduction of ill health and death cause by mental illness... which is related to physical and social environments', and any attempts to raise awareness of the issues that surround sexual health, which the government recognises are complex and not restricted to the control of disease'. We feel it is important to recognise that there needs to be a willingness to address and discuss attitudes and behaviour in what are very sensitive areas.
Key Points
- Knowledge of the effects and side-effects of drugs used for gender re-assignment comes from experience in other fields.
- Pharmacological therapy involves suppression of the characteristics of the original gender and enhancement of characteristics of the adopted gender.
- Monitoring of response relies heavily on the patient's impressions. Objective measures are less helpful.
- Measurement of relevant hormone levels and other blood tests may forewarn of adverse effects of treatment.
Authors
- David Bromham MB BS MRCS FRCOG - Senior Lecturer in Obstetrics; Gynaecology
- Rosemary Pearson RN RM BSc - Honorary Research Nurse, St James's University Hospital, Leeds.
British Journal of Sexual Medicine, September/October 1996.
References
- Siddle NC, Knight MA (eds). Managing the menopause: a practical guide to HRT. Oxted:Medical Communication Series, 1991; 63:54-56.
- Dalton ME, Bhabra K, Bromham DR. Transdermal patches for male and female gender re-assignment. In: Genazzanni AR, Petraglia F, Volpe A, Facchinetti F (eds) Recent research of gynaecological endocrinology. Carnforth: Parthenon Publishing Group, 1989;2:263-278.
- Goh HH, Ratnam SS. Effects of oestrogens on prolactin secretion in transsexual subjects. Arch Sex Behav 1990; 19:507-526.
- Committee on Safety of Medicines, Hepatic Reactions with cyproterone acetate (Cyprostat, Androcur). Curr Probl Pharmacovig 1995;21:1
- Freidl KE, Reappraisal of the health risks associated with the use of high doses of oral and injectable androgenic steroids. NIDA Res Monogr 1990;102:142-177
- Katz DL, Pope HG, Anabolic-androgenic steroid-induced mental status changes, NIDA, Res Monogr 1990;102:215-223
- Ferenchick GS. Are androgenic steroids thrombogenic? N Engl J Med 1990; 322:476.
- Aitkenhead AR. Prudence with the pill. J Med Defence Union 1990; 6:62-64
- The Secretary of State for Health. Health of the Nation, London: HMSO, 1991.
Comments
comments powered by Disqus