Abstract
The concern that postmenopausal hormone replacement therapy (HRT) may cause cancer of the breast has generated much research in epidemiology, endocrinology, and tumor cell biology. The recognition that naturally occurring 17beta-estradiol is a weak genotoxic and mutagenic carcinogen provides a plausible background for the association of breast cancer with HRT. However, because of the small anticipated effect and several confounding factors, the epidemiology of this association is complex. The consensus at this writing is that long-term HRT (>10 years) is associated with an increased risk of breast cancer, which, on average, is equivalent to the risk associated with delaying menopause for the same period of time. The particular risk depends on the duration and probably the dose to which the individual woman is exposed, as well as on a number of predisposing environmental and genetic factors.
One clinical implication of the data reviewed here is that the dosage of HRT chosen should be the lowest that produces the desired effect. The use of HRT in women with a history of breast cancer is also addressed. Low-dose estrogen together with a selective estrogen receptor modulator to protect the breast may be a treatment option for women with severe symptoms of estrogen deficiency.
by Howard S. Jacobs MD, FRCP, FRCOG
Introduction
Evidence of a link between breast cancer and postmenopausal hormone replacement therapy (HRT) has been steadily accruing over the past few years. Available data indicate that there is an increased risk of breast cancer attributable to long-term HRT (> 10 years) that is at least equivalent to the risk associated with delaying menopause for a similar duration of time. This conclusion is intuitively plausible because it has been known for many years that an early menopause protects against breast cancer and that the purpose of HRT is to reverse the endocrine deficit of menopause.
Because most of the epidemiological information has been acquired from observational rather than experimental studies, the field is bedeviled by problems of bias and confounding. Given the probable relation between the risk of cancer and the dose and duration of treatment, I make some broad recommendations concerning prescription of treatment. I also review the difficult therapeutic problem presented by women with a history of breast cancer whose symptoms of hormone deficiency are so severe that it becomes appropriate to consider treatment with estrogen.
Risk of Breast Cancer
Endogenous Oestrogens
Late menopause has long been associated with an increased risk of breast cancer and, conversely, early menopause with a reduced risk of breast cancer.(1) This observation is consistent with the notion that prolonged exposure to endogenous estrogen is an adverse risk factor.(2) For every 1-year increase in age at menopause, there is an approximate 3% increase in the risk of breast cancer.(3) The exact figure depends on the age at which the cancer is diagnosed; for women aged 50-59 years, it is as high as 4% per year. The incidence of breast cancer in relation to age and the time of menopause is shown in Figure 1.(4) In contrast to the non-hormone-dependant colorectal cancer, the increased incidence rate of breast cancer slows after age 50 years (Figure 2). As expected, postmenopausal women have a lower risk of breast cancer than premenopausal women of the same age and childbearing pattern.
After menopause, extraglandular conversion of androgens to estrogens in fat tissue is the primary source of circulating estrogens. The 2 most important determinants of the extraglandular estrogen production rate are the availability of substrate and the woman's body weight. Serum estrogen concentrations increase with body weight: the mean concentration in postmenopausal women whose body mass index (BMI) is equal to or greater than 29 kg/m2 being double that of women with a BMI of less than 21 kg/m2.(5) The relative risk of breast cancer in postmenopausal women increases with body weight,(6] rising by 3.1% per kg/m2.(3)
Several studies have reported on the relationship between the risk of postmenopausal breast cancer and hormone levels, as indexed by blood concentrations of estrogens. A recent systematic review(7) assessed 29 epidemiologic papers – in the 6 prospective studies, the mean serum estradiol concentration in women who subsequently developed breast cancer was 15% higher than the concentration in those who remained cancer free. These results have been confirmed in 2 further reports.(8,9) It seems, therefore, that even a single measurement of serum estradiol concentration in a postmenopausal woman gives some prediction of the risk of breast cancer developing over the next few years. Although there is some stability in serum estrogen concentrations in postmenopausal women,(10) the investigation of hormone concentrations has been complemented by studies in which the risk of breast cancer has been related to markers of hormone action. Such markers represent the impact of a long period of exposure to estrogen. A reduced risk of breast cancer has been reported in postmenopausal women with a history of osteoporotic fracture,(11,12) conversely, it has also been found that, as bone mineral density increased, the risk of breast cancer increased.(13)
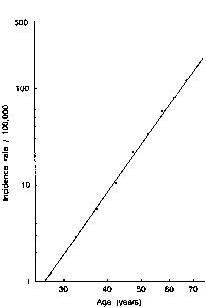
Figure 1. Log-log plot of age specific incidence rates for breast cancer (per 100,000) in US white women, 1969-1971. From Cutler SY, Young JL. Third National Cancer Survey: incidence data. Natl Cancer Inst Monogr. 1975;41. © 1975 Oxford University Press. Reproduced with permission.
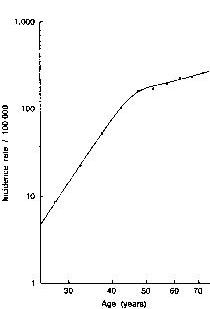
Figure 2. Log-log plot of age specific incidence rates for colorectal cancer (per 100,000) in US white women, 1969-1971. Note the shape of the curve for a non-hormone dependant malignancy (Fig 2) compared with the inflection at the age of menopause in women with breast cancer (Fig 1). From Cutler SY, Young JL. Third National Cancer Survey: incidence data. Natl Cancer Inst Monogr. 1975;41. © 1975 Oxford University Press. Reproduced with permission.
Exogenous Oestrogens
Zumoff(14) cited 69 epidemiologic reports published between 1941 and 1996 that concerned the effect of hormone replacement on the risk of breast cancer. He reported that 27 studies showed a slight increase, 32 showed no difference, and 10 a slight decrease in the risk of breast cancer in women taking HRT. There have been 8 meta-analyses: 3 showed no difference,(15-17) and 5, including the most recent, showed an increase in risk from long-term use.(18-22) However, the most powerful epidemiologic assessment has been the re-analysis of published data by Beral and her colleagues in the Collaborative Group on Hormonal Factors in Breast Cancer.(3) The group collected individual data on 52,705 women with and 108,411 women without breast cancer from 51 epidemiologic studies performed in 21 countries. The information was checked and analyzed centrally. The analysis was based on 53,865 postmenopausal women whose age at menopause was known, of whom 17,830 (33%) had used HRT at some time.
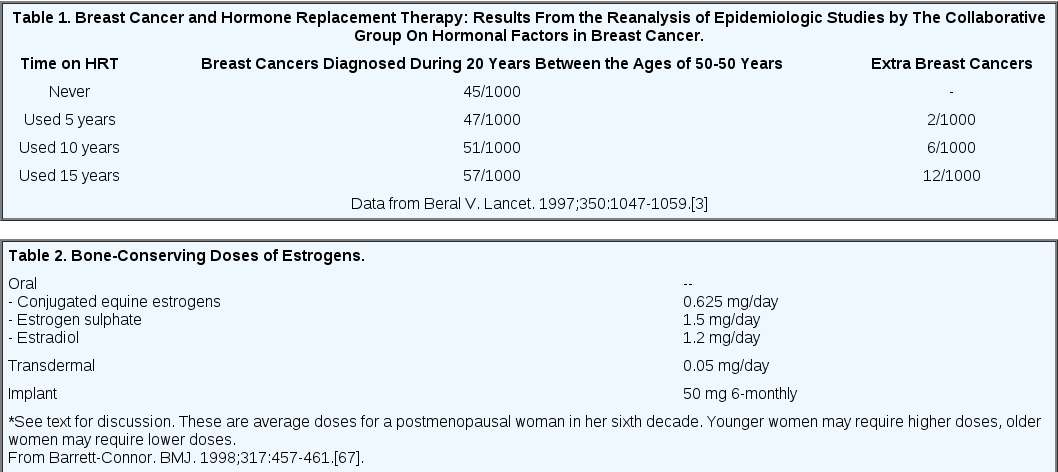
The main finding of the re-analysis was that there was a statistically significant increase in the relative risk of breast cancer for current or recent (past 1-4 years) users of HRT, which increased with duration of use.(3) There was an important interaction with body weight – the relative risk of cancer developing during HRT declined with increasing body weight. Overall, the risk of having breast cancer diagnosed increased by 2.3% a year for each year of use (average duration of use 11 years). The re-analysis showed no increased risk of breast cancer in past users (> 5 years previously); 5 years after discontinuing HRT, there was no significant excess of breast cancer. The cumulative numbers of cancer cases attributable to HRT are shown in Table 1.
This very large data set, which represented about 90% of the published epidemiologic evidence available at the time, permitted both stratification and analysis for confounding and bias. Failure to take into account time that had elapsed since menopause would have resulted in a substantial underestimate of the risk of breast cancer associated with the use of HRT and the significantly increased risk with duration of use would not have been detected. Failure to stratify by body mass also underestimates risk. Thus, it appears that HRT may have its largest impact in women who, by virtue of their low body weight, are least likely to develop breast cancer spontaneously. If a negative mammogramis required before estrogens are prescribed, the risk of developing breast cancerwill again be underestimated. Selection bias also results in underestimated risk if estrogens are withheld from women who are at increasedrisk for breast cancer (ie, those with a positive family history) or selectively prescribed to women at reduced risk(ie, those with an early menopause). Surveillance bias is suggested by reports in which women with estrogen-associated breast cancer had a betterprognosis than women with breast cancer who were not being treatedwith estrogen,(23-25) although a recent study has reported an increase in fatal breastcancer as well.(26) Differences in surveillance may also bias results in the other direction: ifwomen who take estrogen are more closely evaluated, as is likely, the risks may appear falsely high. It does seem, however, that most of the biases in these observational studies operate to underestimate the true risk of a woman on HRT developing breast cancer.
Information about which hormonal preparations were used was available to the Collaborative Group for 39% of the study population – 80% had used preparations mostly containing estrogen alone. There was, however, insufficient information to determine whether addition of progestogen to treatment with estrogen had a deleterious effect. Results from the Nurses Health Study had indicated that, with respect to breast cancer, progestins conferred no protection from the risk of breast cancer associated with estrogen treatment.(27) Two studies published this year have, however, shown that the combination of estrogen with progestin is associated with a greater risk of breast cancer than treatment with estrogen alone. One study, which included 2082 cases of breast cancer identified from 29 screening centers in the United States, reported that treatment with the combination increased breast cancer risk beyond that associated with estrogen alone.(28) The second, a population based case-controlled study of 1897 women with breast cancer and 1637 controls, described a 10% higher incidence of breast cancer for each 5 years of combination estrogen/progestin use.(29) The relative risk was substantially higher for combined treatment (odds ratio (OR], 1.24; 95% confidence interval (CI] -1.07-1.45) compared with treatment with estrogen alone (OR, 1.06; 95% CI 0.97-1.15). The risk estimate was higher for sequential than for continuous use of progestin, although this difference did not achieve statistical significance.
These results raise questions about the contributions of the 2 components of HRT regimens. There is now a substantial body of data to show that the natural hormone 17beta estradiol can induce multiple forms of DNA damage in vitro and in laboratory animals.(30) Several of these estrogen-induced DNA lesions have been detected in human tissue. In addition, estradiol induces gene mutations, albeit at low frequency. The data suggest that estrogen is a weak carcinogen and mutagen capable of inducing a low frequency of genetic lesions and subsequent tumor formation.(30 ) Tumors may then develop as a result of receptor-mediated estrogen-stimulated proliferation of damaged cells (Figure 3). In the breast, and in contrast to the uterus, progesterone also has a proliferative effect, as reviewed by Pike and colleagues.(4) These investigators have developed an "estrogen augmented by progestogen," model which seems most helpful for understanding the role of the 2 classes of hormone in the epidemiology and prevention of breast cancer.(31)
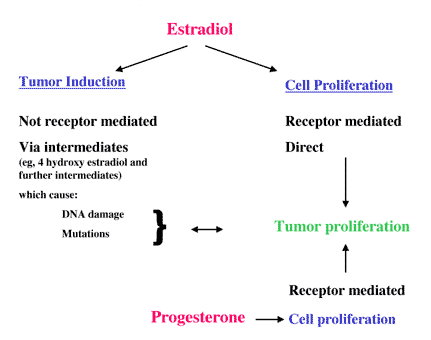
Figure 3. Proposed carcinogenic actions of estradiol and progesterone. Data from Liehr JG. Endocr Rev. 2000;21:40-54.
The excess of cancer cases, as reported by the Collaborative Group(3) above, was largely due to localized disease. Two other studies had reported a higher risk of in situ than invasive cancer associated with HRT.[32,33) A recent case series, however, described invasive cancers in women on HRT that were less aggressive than those seen in women not on HRT.(34) More persuasively, a positive relationship between the incidence of invasive breast cancer with a favorable histology and duration of estrogen use was found in a recent prospective cohort study,(35) a relationship that was stronger for current users than for past users. These results are consistent with reports that the prognosis in women with breast cancer developing during HRT is better than in women not taking estrogen,(23-25) although it is uncertain how much of this difference is attributable to surveillance bias.
Although correlations of HRT use with steroid receptor status have been described,(36,37) in most of the studies no significant differences in receptor profiles between HRT users and nonusers have been detected.(38) The significance of these results is uncertain because, until recently, most studies used the dextran-coated charcoal assay,(39) which detects unoccupied receptors only, and in the presence of exogenous estrogen one might expect the sites to be occupied. The more widespread use of monoclonal antibody-based methods may shed light on possible, but as yet undetected, differences between receptor profiles of HRT users and nonusers.
Individual Susceptibility
Because a small proportion of women exposed to HRT develops breast cancer, much research is currently focused on discovering factors that may explain individual susceptibility. Some of these factors are related to observable changes in the breast, some to genetic factors that may determine hormone levels and some to environmental influences, such as alcohol consumption.
An increase in breast density can be detected by mammography in 15% to 50% of women on HRT.(40) Greater breast density has been independently associated with a doubling of the risk of breast cancer,(41) and the risk persists for up to 9 years postmammography, which suggests that masking of breast cancer in denser tissue is not the sole cause of the observed association.(22) The results are consistent with estrogen stimulation of epithelial cell proliferation in the breast.
Genetic mechanisms that may help to explain some of the differences in endogenous hormone levels have recently been investigated. A polymorphism of 1 of the genes that encodes enzymes responsible for adrenal and ovarian production of sex steroids (CYP17) has been described.(42) Although it is not involved in genesis of the polycystic ovary syndrome, as originally thought, CYP17 may be important in determining postmenopausal hormone concentrations. Thus, in 1 study, postmenopausal women with the CYP17A2/A2 genotype had significantly higher levels of estrone (+14.3%) and dehydroepiandrosterone (+14.4%) than women with the A1/A1 genotype.(43) Similar elevations of mean serum estradiol and androstenedione concentrations were also found, however, they were not significant. In a separate study, women who had the CYP17 A2/A2 genotype were about half as likely as women with the A1/A1 genotype to be current users of HRT.(44) This result is consistent with the notion that women with the lowest endogenous hormone levels are most likely to choose hormone treatment. Conversely, those with the highest endogenous hormone levels (who, as discussed above, are most at risk for breast cancer) are likely to be underrepresented among users of HRT, thus causing a statistical underestimation of the true risk associated with estrogen treatment.
This discussion has focused thus far on the production of hormones. For some years, Bradlow and colleagues(45) and Fishman and colleagues(46) have emphasized the importance of the relationship between the metabolism of estrogens and the risk for breast cancer. Estradiol metabolism is predominantly oxidative, initially reversibly to estrone, then irreversibly by 1 of 2 pathways. The first is by 2-hydroxylation to form the nonestrogenic catechol, 2-hydroxyestrone; the second is by 16alpha-hydroxylation to produce 16alpha-hydroxyestrone and then estriol (both of which are estrogenic). The effect of secreted or administered estrogen depends on the balance between these metabolic pathways.(14) Increased 16alpha-hydroxylation in women with breast cancer(47) has been confirmed recently in both a case-control(48) and a prospective cohort study.(49) In the prospective study by Meilahn and associates,(49) postmenopausal women at baseline who went on to develop breast cancer showed an approximately 15% lower ratio of 2:16alpha-hydroxyoestrone than matched control subjects.
Genetic factors are important in determining the direction of this metabolic pathway,(50) but body weight is also relevant – thin women (at lower risk of breast cancer) produce more catechol metabolites, overweight women (at greater risk) produce more 16alpha metabolites.(51) It may be possible to alter the direction of this metabolic pathway by administering relatively simple compounds,(52) which may result in the development of chemoprevention strategies for those most at risk.(53)
Alcohol Consumption
A large case-control study in 1977 first reported on the association between alcohol consumption and an increased risk of breast cancer.(54) Since then, the association has been examined in more than 50 epidemiologic studies.(55) A meta-analysis of 28 case-controlled and 10 cohort studies showed a dose-response relationship between the quantity of alcohol consumed and the risk of breast cancer.(56) Women who had a daily intake of 26 g of ethanol had a 1.24 (95% CI, 1.15-1.34) risk for breast cancer compared with those who had no ethanol intake. The risk associated with 1 alcoholic drink per day (approximately 13 g of alcohol) was about 10% greater than in nondrinkers. There was marked variation between studies but the association was strongest in countries with the highest per capita intake. A pooled analysis of 6 cohort studies from Canada, the Netherlands, Sweden, and the United States (comprising 322,647 women, of whom, 4335 developed invasive breast cancer) showed a linear increase in breast cancer incidence with increasing alcohol consumption.(57)
The explanation for this association is not certain, but an endocrine mechanism may provide the link. A recent review(58) noted an increase in serum estradiol concentrations in response to alcohol consumption in 2 of 6 studies of untreated postmenopausal women. Two studies of women on HRT showed an increase of serum estradiol concentrations after a single but substantial dose of alcohol. In a study of women receiving HRT via a dermal patch, the increase was modest (22% [n = 7]).(59) In a separate report on women taking oral estrogen, the mean increase of estradiol concentrations was striking (300% [n = 12]).(60) The results are consistent with an effect of alcohol on splanchnic metabolism of estrogens.
Several investigators have described the receptor status of breast cancers in relation to alcohol consumption. A recent report by Enger and associates,(61) which describes the largest series to date, provides a valuable summary of the literature. On the basis of their own data, the authors concluded that ingestion of alcohol preferentially increased the risk of estrogen (and progesterone) receptor-positive breast cancer in postmenopausal women. Contrary findings have, however, also been reported.(62) Two groups have reported that the greatest risk of breast cancer occurs in postmenopausal women on HRT who consume alcohol.(63,64) This link has been stressed in the endocrine literature,(65) although the extent to which the association should be attributed to confounding is unresolved.(66) If confirmed, the association would clearly be important because alcohol consumption is common and, in contrast to most of the currently recognized risk factors, it can be modified.
Implications for HRT
Until the results of randomized controlled clinical trials become available, the reanalysis by the Collaborative Group(3) provides the best estimate of the average risk a woman takes when she begins oral estrogen replacement therapy. The epidemiologic data shown in Table 2, however, need to be modified according to the individual's endogenous risk factors. The question regarding the optimal duration of therapy will remain unresolved until more information about the prevention of cardiovascular disease and Alzheimer's disease is available from randomized controlled trials. Only then will it be possible to make accurate risk-benefit analyses. One practical implication of the extensive work reviewed here does seems to be for women to use the lowest dose of estrogen that is effective. As discussed by Barrett-Connor,(67) currently advised doses of estrogen and progestin were designed to prevent bone loss and endometrial cancer, respectively. However, the current recommendations are not based on studies of a wide range of doses. Recent reports have indicated that for many women, a daily dose of conjugated estrogen (or its equivalent) as low as 0.3 mg together with 1 g of dietary calcium is sufficient for the prevention of osteoporosis.(68,69)
Estradiol levels within the physiologic range, control of symptoms, and prevention of bone rarefaction can be achieved with subcutaneous estrogen implants of 25 mg given every 6-9 months.(70,71) Nonetheless, most clinicians who advocate implant use larger doses.(72) Garnet and colleagues,(73) who administered implants containing 50 or 75 mg of estradiol every 6 months reported a mean serum estradiol concentration of 767 pmols/L in 1388 women seen in 1 clinic during 1 year; the range was wide, with only 17.1% having a concentration below 500 pmols/L. Three percent of the women had serum levels exceeding 1750 pmols/L. Apart from the extraordinarily prolonged duration of action of these implants – gonadotropin concentrations may be suppressed for up to 3 years after implantation of 100-mg implants(74) and endometrial stimulation may continue for even longer(75) – one cannot be optimistic about the effects of these very high estrogen levels on the breast. Moreover, it appears that a proportion of women develop a need for reimplantation at shorter and shorter intervals.(73,76) It appears that their physicians are not adhering to the axiom that estrogens should be prescribed in the lowest effective dose for specific indications, rather, they are inserting estrogen implants for psychological rather than endocrinologic reasons.(77,78)
Should Women with a History of Breast Cancer Use HRT?
As a result of advances in diagnosis and treatment, there is a large and growing cohort of women who can look forward to many years of survival after treatment for breast cancer. Many are postmenopausal at the time of diagnosis; in a significant proportion of premenopausal cases cytotoxic treatment causes ovarian damage sufficient to precipitate menopause. These circumstances, combined with the increasing concern of physicians and patients about the impairment of quality of life caused by estrogen deficiency, has lead to a reappraisal of the traditional advice that women with breast cancer should avoid estrogen therapy. It has been argued that it is now time to rethink the role of HRT in women with a history of breast cancer.(79)
At present, there are no randomized controlled trials on which to base decisions and, given the understandable reluctance of patients to enter such trials, it is likely to be several years before robust data become available. At present, the only available data are from case series. Results from 8 series(80-87) totaling 501 patients with cancers at various stages showed that 37 (7.4%) of these patients suffered a recurrence while on treatment. A separate report described 4 patients who developed metastatic breast cancer while on HRT; in each of whom regression followed withdrawal of treatment.(88)
Opinion is understandably divided on the safety of HRT after treatment of breast cancer and where possible nonhormonal treatment options are favored.(89) On the other hand, for some women quality of life is so impaired by estrogen deficiency that with holding HRT is unreasonable. Obviously, the indication for such treatment should be severe symptoms of estrogen deficiency rather than the prevention of long-term complications. Treatment should be preceded by careful explanation and discussion; the dosage should be the lowest that resolves symptoms. Combined treatment with estrogen and tamoxifen has been described(89,90) on the basis that tamoxifen is effective in preventing breast cancer in premenopausal women, in whom estrogen levels are higher than in postmenopausal women on low-dose HRT. An alternative approach to be explored would be the combination of estrogen with raloxifene, the latter chosen because its estrogen antagonism extends to the uterus,(91) thus reducing the risk of endometrial stimulation and therefore the need for cotreatment with progestins.
References
- Hulka BS, Stark AT. Breast cancer: cause and prevention. Lancet. 1995;346:883-887.
- Colditz GA. Relationship between estrogen levels, use of hormone replacement therapy, and breast cancer. J Natl Cancer Inst. 1998;90:814-823.
- Beral V. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Collaborative Group on Hormonal Factors in Breast Cancer [published erratum appears in Lancet 1997;350:1484]. Lancet. 1997;350:1047-1059.
- Pike MC, Spicer DV, Dahmoush L, Press MF. Estrogens, progestogens, normal breast cell proliferation, and breast cancer risk. Epidemiol Rev. 1993;15:17-35.
- Hankinson SE, Willett WC, Manson JE, et al. Alcohol, height, and adiposity in relation to estrogen and prolactin levels in postmenopausal women. J Natl Cancer Inst. 1995;87:1297-1302.
- Ballard-Barbash R, Swanson CA. Body weight: estimation of risk for breast and endometrial cancers. Am.J.Clin.Nutr. 1996;63(suppl 3):437S-41S.
- Thomas HV, Reeves GK, Key TJ. Endogenous estrogen and postmenopausal breast cancer: a quantitative review. Cancer Causes Control. 1997;8:922-928.
- Cauley JA, Lucas FL, Kuller LH, Stone K, Browner W, Cummings SR. Elevated serum estradiol and testosterone concentrations are associated with a high risk for breast cancer. Study of Osteoporotic Fractures Research Group. Ann Intern Med. 1999;130(4 pt 1):270-277.
- Hankinson SE, Willett WC, Manson JE, et al. Plasma sex steroid hormone levels and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 1998;90:1292-1299.
- Key TJ. Serum estradiol and breast cancer risk. Endocrine-Related Cancer. 1999;6:175-180.
- Olsson H, Hagglund G. Reduced cancer morbidity and mortality in a prospective cohort of women with distal forearm fractures. Am J Epidemiol. 1992;136:422-427.
- Persson I, Adami HO, McLaughlin JK, Naessen T, Fraumeni JFJ. Reduced risk of breast and endometrial cancer among women with hip fractures (Sweden). Cancer Causes Control. 1994;5:523-528.
- Kuller LH, Cauley JA, Lucas L, Cummings S, Browner WS. Sex steroid hormones, bone mineral density, and risk of breast cancer. Environ Health Perspect. 1997;105(suppl 3):593-599.
- Zumoff B. Biological and endocrinological insights into the possible breast cancer risk from menopausal estrogen replacement therapy. Steroids. 1993;58:196-204.
- Armstrong BK. Estrogen therapy after the menopause – boon or bane? Med J Aust. 1988;148:213-214.
- Dupont WD, Page DL. Menopausal estrogen replacement therapy and breast cancer. Arch Intern Med. 1991;151:67-72.
- Gambrell RDJ. Hormone replacement therapy and breast cancer risk. Arch Fam Med. 1996;5:341-348.
- Steinberg KK, Thacker SB, Smith SJ, et al. A meta-analysis of the effect of estrogen replacement therapy on the risk of breast cancer [published erratum appears in JAMA 1991;266:1362]. JAMA. 1991;265:1985-1990.
- Grady D, Ernster V. Invited commentary: does post menopausal hormone therapy cause breast cancer? Am J Epidemiol. 1991;134:1396-1400.
- Sillero-Arenas M, Delgado-Rodriguez M, Rodrigues-Canteras R, Bueno-Cavanillas A, Galvez-Vargas R. Menopausal hormone replacement therapy and breast cancer: a meta- analysis. Obstet Gynecol. 1992;79:286-294.
- Colditz GA, Egan KM, Stampfer MJ. Hormone replacement therapy and risk of breast cancer: results from epidemiologic studies. Am J Obstet Gynecol. 1993;168:1473-1480.
- Barrett-Connor E, Grady D. Hormone replacement therapy, heart disease, and other considerations. Annu Rev Public Health. 1998;19:55-72.
- Persson I, Yuen J, Bergkvist L, Schairer C. Cancer incidence and mortality in women receiving estrogen and estrogen-progestin replacement therapy – long-term follow-up of a Swedish cohort. Int J Cancer. 1996;67:327-332.
- Willis DB, Calle EE, Miracle-McMahill HL, Heath CWJ. Estrogen replacement therapy and risk of fatal breast cancer in a prospective cohort of postmenopausal women in the United States. Cancer Causes Control. 1996;7:449-457.
- Jernstrom H, Frenander J, Ferno M, Olsson H. Hormone replacement therapy before breast cancer diagnosis significantly reduces the overall death rate compared with never-use among 984 breast cancer patients. Br J Cancer. 1999;80:1453-1458.
- Grodstein F, Stampfer MJ, Colditz GA, et al. Postmenopausal hormone therapy and mortality. N Engl J Med. 1997;336:1769-1775.
- Colditz GA, Hankinson SE, Hunter DJ, et al. The use of estrogens and progestins and the risk of breast cancer in postmenopausal women. N Engl J Med. 1995;332:1589-1593.
- Schairer C, Lubin J, Troisi R, Sturgeon S, Brinton L, Hoover R. Menopausal estrogen and estrogen-progestin replacement therapy and breast cancer risk. JAMA. 2000;283:485-491.
- Ross RK, Paganini-Hill A, Wan PC, Pike MC. Effect of hormone replacement therapy on breast cancer risk: estrogen versus estrogen plus progestin. J Natl Cancer Inst. 2000;92:328-332.
- Liehr JG. Is estradiol a genotoxic mutagenic carcinogen? Endocr Rev. 2000;21:40-54.
- Key TJ, Pike MC. The role of estrogens and progestagens in the epidemiology and prevention of breast cancer. Eur J Cancer Clin Oncol. 1988;24:29-43.
- Longnecker MP, Bernstein L, Paganini-Hill A, Enger SM, Ross RK. Risk factors for in situ breast cancer. Cancer Epidemiol Biomarkers Prev. 1996;5:961-965.
- O'Connor IF, Shembekar MV, Shousha S. Breast carcinoma developing in patients on hormone replacement therapy: a histological and immunohistological study. J Clin Pathol. 1998;51:935-938.
- Holli K, Isola J, Cuzick J. Low biologic aggressiveness in breast cancer in women using hormone replacement therapy. J Clin Oncol. 1998;16:3115-3120.
- Gapstur SM, Morrow M, Sellers TA. Hormone replacement therapy and risk of breast cancer with a favorable histology: results of the Iowa Women's Health Study. JAMA. 1999;281:2091-2097.
- Jones C, Ingram D, Mattes E, Hahnel R. The effect of hormone replacement therapy on prognostic indices in women with breast cancer. Med J Aust. 1994;161:106-110.
- Bonnier P, Bessenay F, Sasco AJ, et al. Impact of menopausal hormone-replacement therapy on clinical and laboratory characteristics of breast cancer. Int J Cancer. 1998;79:278-282.
- Cobleigh MA, Norlock FE, Oleske DM, Starr A. Hormone replacement therapy and high S phase in breast cancer. JAMA. 1999;281:1528-1530.
- Habel LA, Stanford JL. Hormone receptors and breast cancer. Epidemiol Rev. 1993;15:209-219.
- Greendale GA, Reboussin BA, Sie A, et al. Effects of estrogen and estrogen-progestin on mammographic parenchymal density. Postmenopausal Estrogen/Progestin Interventions (PEPI) Investigators. Ann Intern Med. 1999;130(4 pt 1):262-269.
- Warner E, Lockwood G, Tritchler D, Boyd NF. The risk of breast cancer associated with mammographic parenchymal patterns: a meta-analysis of the published literature to examine the effect of method of classification. Cancer Detect Prev. 1992;16:67-72.
- Techatraisak K, Conway GS, Rumsby G. Frequency of a polymorphism in the regulatory region of the 17 alpha-hydroxylase-17,20-lyase (CYP17) gene in hyperandrogenic states. Clin Endocrinol (Oxf). 1997;46:131-134.
- Haiman CA, Hankinson SE, Spiegelman D, et al. The relationship between a polymorphism in CYP17 with plasma hormone levels and breast cancer. Cancer Res. 1999;59:1015-1020.
- Feigelson HS, McKean-Cowdin R, Pike MC, et al. Cytochrome P450c17alpha gene (CYP17) polymorphism predicts use of hormone replacement therapy. Cancer Res. 1999;59:3908-3910.
- Bradlow HL, Telang NT, Sepkovic DW, Osborne MP. 2-hydroxyestrone: the 'good' estrogen. J Endocrinol. 1996;150(suppl):S259-S265.
- Fishman J, Osborne MP, Telang NT. The role of estrogen in mammary carcinogenesis. Ann NY Acad Sci. 1995;768:91-100.
- Fishman J, Schneider J, Hershcope RJ, Bradlow HL. Increased estrogen-16 alpha-hydroxylase activity in women with breast and endometrial cancer. J Steroid Biochem. 1984;20:1077-1081.
- Kabat GC, Chang CJ, Sparano JA, et al. Urinary estrogen metabolites and breast cancer: a case-control study. Cancer Epidemiol Biomarkers Prev. 1997;6:505-509.
- Meilahn EN, De Stavola B, Allen DS, et al. Do urinary estrogen metabolites predict breast cancer? Guernsey III cohort follow-up. Br J Cancer. 1998;78:1250-1255.
- Taioli E, Bradlow HL, Garbers SV, et al. Role of estradiol metabolism and CYP1A1 polymorphisms in breast cancer risk. Cancer Detect Prev. 1999;23:232-237.
- Fishman J, Boyar RM, Hellman L. Influence of body weight on estradiol metabolism in young women. J Clin Endocrinol Metab. 1975;41:989-991.
- Hershcopf RJ, Bradlow HL. Obesity, diet, endogenous estrogens, and the risk of hormone-sensitive cancer. Am J Clin Nutr. 1987;45(suppl 1):283-289.
- Wong GY, Bradlow L, Sepkovic D, Mehl S, Mailman J, Osborne MP. Dose-ranging study of indole-3-carbinol for breast cancer prevention. J Cell Biochem Suppl. 1997;28-29:111-116.
- Williams RR, Horm JW. Association of cancer sites with tobacco and alcohol consumption and socioeconomic status of patients: interview study from the Third National Cancer Survey. J Natl Cancer Inst. 1977;58:525-547.
- Schatzkin A, Longnecker MP. Alcohol and breast cancer. Where are we now and where do we go from here? Cancer. 1994;74(suppl 3):S1101-S1110.
- Longnecker MP. Alcoholic beverage consumption in relation to risk of breast cancer: meta-analysis and review. Cancer Causes Control. 1994;5:73-82.
- Smith-Warner SA, Spiegelman D, Yaun SS, et al. Alcohol and breast cancer in women: a pooled analysis of cohort studies. JAMA. 1998;279:535-540.
- Purohit V. Moderate alcohol consumption and estrogen levels in postmenopausal women: a review. Alcohol Clin Exp Res. 1998;22:994-997.
- Ginsburg ES, Walsh BW, Gao X, Gleason RE, Feltmate C, Barbieri RL. The effect of acute ethanol ingestion on estrogen levels in postmenopausal women using transdermal estradiol. J Soc Gynecol Investig. 1995;2:26-29.
- Ginsburg ES, Mello NK, Mendelson JH, et al. Effects of alcohol ingestion on estrogens in postmenopausal women. JAMA. 1996;276:1747-1751.
- Enger SM, Ross RK, Paganini-Hill A, Longnecker MP, Bernstein L. Alcohol consumption and breast cancer estrogen and progesterone receptor status. Br J Cancer. 1999;79:1308-1314.
- Gapstur SM, Potter JD, Drinkard C, Folsom AR. Synergistic effect between alcohol and estrogen replacement therapy on risk of breast cancer differs by estrogen/progesterone receptor status in the Iowa Women's Health Study. Cancer Epidemiol Biomarkers Prev. 1995;4:313-318.
- Colditz GA. A prospective assessment of moderate alcohol intake and major chronic diseases. Ann Epidemiol. 1990;1:167-177.
- Gapstur SM, Potter JD, Sellers TA, Folsom AR. Increased risk of breast cancer with alcohol consumption in postmenopausal women. Am J Epidemiol. 1992;136:1221-1231.
- Zumoff B. Alcohol, estrogens, and breast cancer [letter; comment]. J Clin Endocrinol Metab. 1997;82:2378.
- Rosenberg L, Metzger LS, Palmer JR. Alcohol consumption and risk of breast cancer: a review of the epidemiologic evidence. Epidemiol Rev. 1993;15:133-144.
- Barrett-Connor E. Hormone replacement therapy. BMJ. 1998;317:457-461.
- Ettinger B, Genant HK, Cann CE. Postmenopausal bone loss is prevented by treatment with low-dosage estrogen with calcium. Ann Intern Med. 1987;106:40-45.
- Ettinger B, Genant HK, Steiger P, Madvig P. Low-dosage micronized 17 beta-estradiol prevents bone loss in postmenopausal women. Am J Obstet Gynecol. 1992;166:479-488.
- Owen EJ, Siddle NC, McGarrigle HT, Pugh MA. 25 mg estradiol implants – the dosage of first choice for subcutaneous estrogen replacement therapy? Br J Obstet Gynaecol. 1992;99:671-675.
- Holland EF, Leather AT, Studd JW. The effect of 25-mg percutaneous estradiol implants on the bone mass of postmenopausal women. Obstet Gynecol. 1994;83:43-46.
- Studd JW, Smith RN. Estradiol and testosterone implants. Baillieres Clin Endocrinol Metab. 1993;7:203-223.
- Garnett T, Studd JW, Henderson A, Watson N, Savvas M, Leather A. Hormone implants and tachyphylaxis. Br J Obstet Gynaecol. 1990;97:917-921.
- Hunter DJ, Akande EO, Carr P, Stallworthy J. The clinical and endocrinological effect of estradiol implants at the time of hysterectomy and bilateral salpingo-oophorectomy. J Obstet Gynaecol Br Commonw. 1973;80:827-833.
- Gangar KF, Fraser D, Whitehead MI, Cust MP. Prolonged endometrial stimulation associated with estradiol implants. BMJ. 1990;300:436-438.
- Gangar K, Cust M, Whitehead MI. Symptoms of estrogen deficiency associated with supraphysiological plasma estradiol concentrations in women with estradiol implants. BMJ. 1989;299:601-612.
- Buckler HM, Kalsi PK, Cantrill JA, Anderson DC. An audit of estradiol levels and implant frequency in women undergoing subcutaneous implant therapy. Clin Endocrinol (Oxf). 1995;42:445-450.
- O'Leary A, Bowen-Simpkins P, Tejura H, Rajesh U. Are high levels of estradiol after implants associated with features of dependence? Br J Obstet Gynaecol. 1999;106:960-963.
- DiSaia PJ. Hormone-replacement therapy in patients with breast cancer. A reappraisal. Cancer. 1993;71(suppl 4):1490-1500.
- Vassilopoulou-Sellin R, Theriault R, Klein MJ. Estrogen replacement therapy in women with prior diagnosis and treatment for breast cancer. Gynecol Oncol. 1997;65:89-93.
- DiSaia PJ, Odicino F, Grosen EA, Cowan B , Pecorelli S, Wile AG. Hormone replacement therapy in breast cancer. Lancet. 1993;342:1232.
- Wile AG, Opfell RW, Margileth DA. Hormone replacement therapy in previously treated breast cancer patients. Am J Surg. 1993;165:372-375.
- Eden JA, Bush T, Nand S, Wren BG. A case control study of combined continuous estrogen-progestin replacement therapy among women with a personal history of breast cancer. Menopause. 1995;2:67-72.
- Powles TJ, Hickish T, Casey S, O'Brien M. Hormone replacement after breast cancer [letter; comment]. Lancet. 1993;342:60-61.
- Stoll BA, Parbhoo S. Treatment of menopausal symptoms in breast cancer patients. Lancet. 1988;1:1278-1279.
- Brewster WR, DiSaia PJ, Grosen EA, McGonigle KF, Kuykendall JL, Creasman WT. An experience with estrogen replacement therapy in breast cancer survivors. Int J Fertil Womens Med. 1999;44:186-192.
- Ursic-Vrscaj M, Bebar S. A case-control study of hormone replacement therapy after primary surgical breast cancer treatment. Eur J Surg Oncol. 1999;25:146-151.
- Dhodapkar MV, Ingle JN, Ahmann DL. Estrogen replacement therapy withdrawal and regression of metastatic breast cancer. Cancer. 1995;75:43-46.
- Santen R, Pritchard K, Burger H. The consensus conference on treatment of estrogen deficiency symptoms in women surviving breast cancer. Obstet Gynecol Surv. 1998;53(suppl 10):S1-S83.
- Chang J, Powles TJ, Ashley SE, et al. The effect of tamoxifen and hormone replacement therapy on serum cholesterol, bone mineral density and coagulation factors in healthy postmenopausal women participating in a randomised, controlled tamoxifen prevention study. Ann Oncol. 1996;7:671-675.
- Khovidhunkit W, Shoback DM. Clinical effects of raloxifene hydrochloride in women. Ann Intern Med. 1999;130:431-439.
Comments
comments powered by Disqus